BACKGROUND
About miRNA
miRNA (microRNA) is short single-stranded non-coding RNA of 20-25 base pairs and has long been considered as “junk RNA” with no physiological role. However, in the 1990s, Professors Ambros and Ruvkun discovered that miRNAs are deeply involved in the regulation of gene expression, particularly in the post-transcriptional control of protein expression, for which they received the Nobel Prize in 2024.
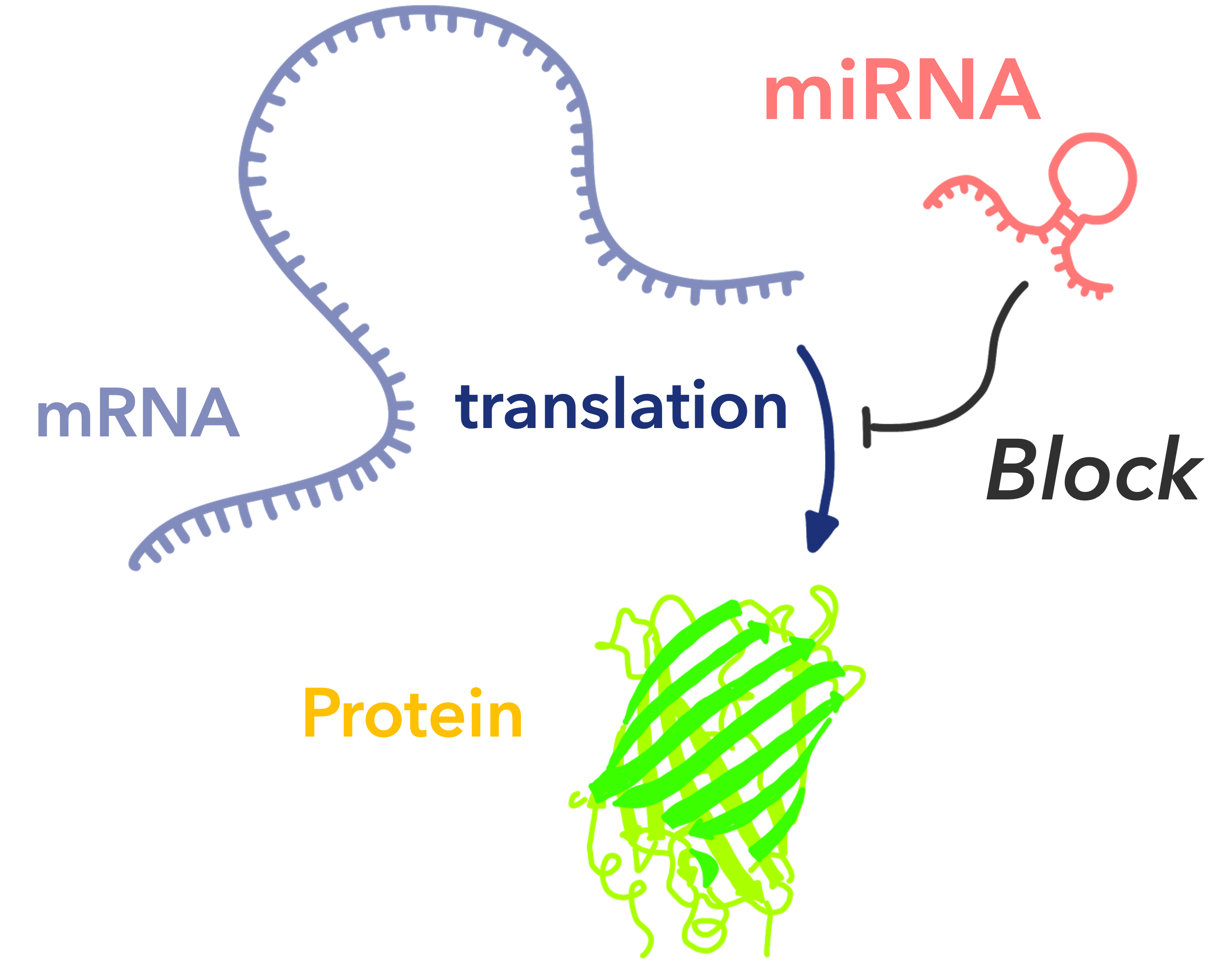
Fig.1 How miRNAs work
This groundbreaking discovery showed that miRNAs are not simply unwanted RNAs, but play important roles in signaling and homeostasis in living organisms, and revolutionized the foundations of conventional biology. This discovery has expanded the field of molecular biology, which had previously focused only on proteins and long RNAs, and opened a new perspective for understanding the precise regulation mechanisms of gene expression.
It is now widely recognized that miRNAs are ubiquitous in most eukaryotes and regulate various physiological processes such as cell development, division, differentiation, and apoptosis through the process of post-transcriptional gene silencing.miRNA dysfunction is implicated in the onset and progression of various diseases, including cancer and neurodegenerative disorders, and research into new diagnostic and therapeutic methods targeting miRNAs is rapidly advancing.
miRNA and Cancer
In cancer research, miRNAs have been the focus of much attention. miRNAs are broadly classified into two types, “cancer-promoting miRNAs” and “cancer-suppressing miRNAs,” which are involved in carcinogenesis and tumor suppression, and are deeply involved in the mechanisms of cancer progression and metastasis. It has been shown that certain miRNAs are overexpressed in cancer cells while other miRNAs are suppressed. This imbalance contributes cancer cells to proliferate, invade, and metastasize. For example, cancer-promoting miRNAs such as miR-21 contribute significantly to the aggressive growth of cancer cells, and suppression of their expression is considered an important target in cancer therapy.
Furthermore, changes in miRNA expression are expected to be a useful biomarker for early diagnosis of cancer. Analysis of miRNA profiles in blood and urine may enable early detection of cancer and monitoring of the risk of recurrence. The potential for such applications has led to active research into the development of non-invasive diagnostic techniques using miRNAs.
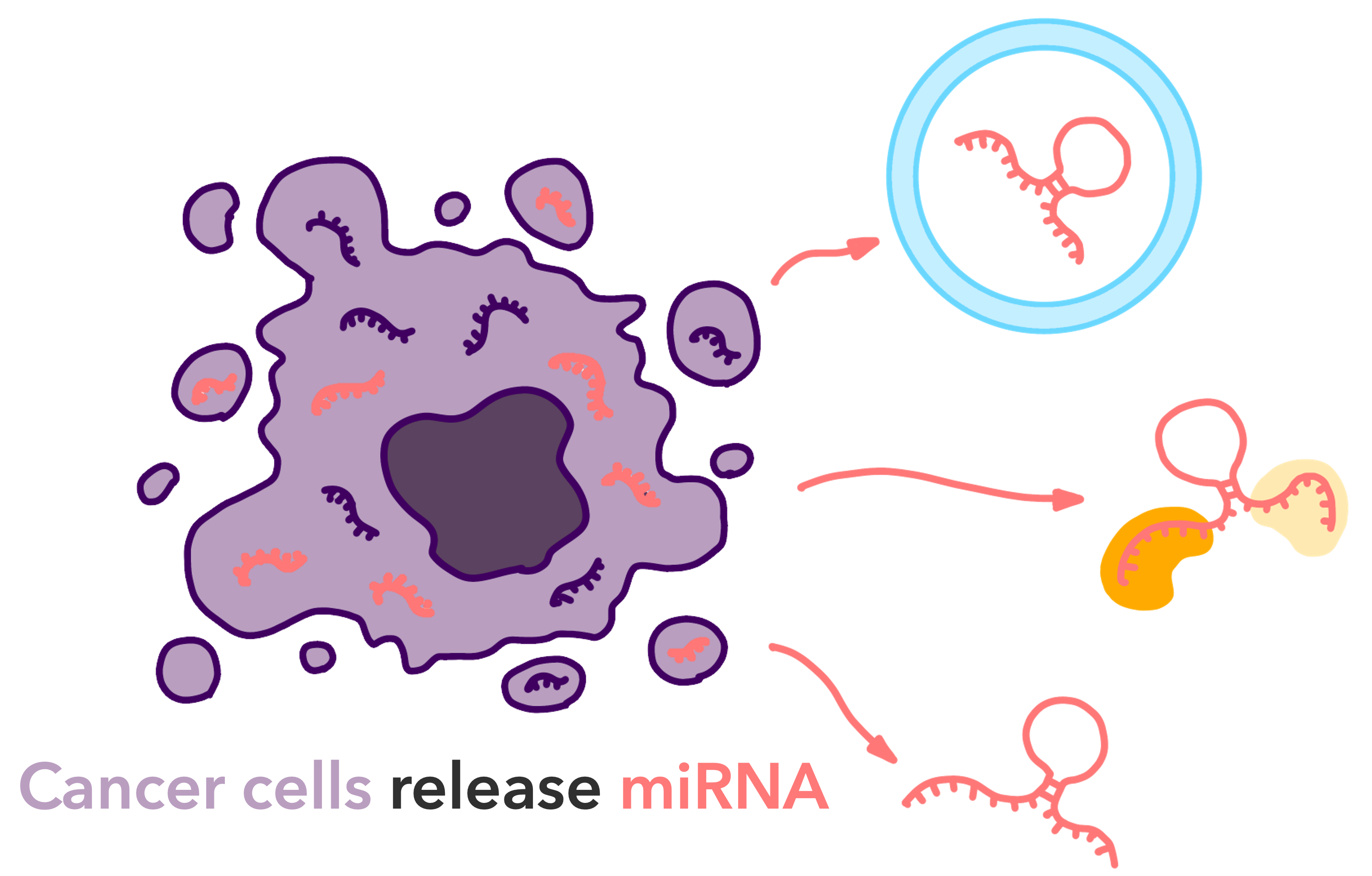
Fig.2 Cancer cells release miRNA
Importance of Unmodified miRNA
Several methods for using miRNAs for the early detection of cancer have been proposed and are being studied for practical application. However, the mechanisms by which miRNAs are involved in life are extremely complex and have not yet been fully elucidated. In addition, miRNAs are very small and in minute quantities, and there are many RNases in the body that degrade miRNAs, so further innovations are needed to stably measure miRNAs.
Among such unstable miRNAs, most of the current studies focus on blood miRNAs that are resistant to RNase.
As a result, some functions of miRNAs in cancer have been elucidated, but these results have biased the research, as “the relationship between cancer and miRNAs that are resistant to RNase” is being studied instead of “the relationship between cancer and miRNAs themselves.
To truly advance research on the relationship between cancer and miRNAs, we focused on Unmodified miRNAs that are not resistant to RNase. The study of unmodified miRNAs in vivo will be very meaningful in terms of their biological functions and the processes of their production and degradation, which have yet to be elucidated. In the course of their research, we will focus on miRNAs that show RNase resistance and the common characteristics of the cells that produce them, and we expect that this research will help establish completely new diagnostic devices and therapies in areas where current miRNA knowledge alone is insufficient.
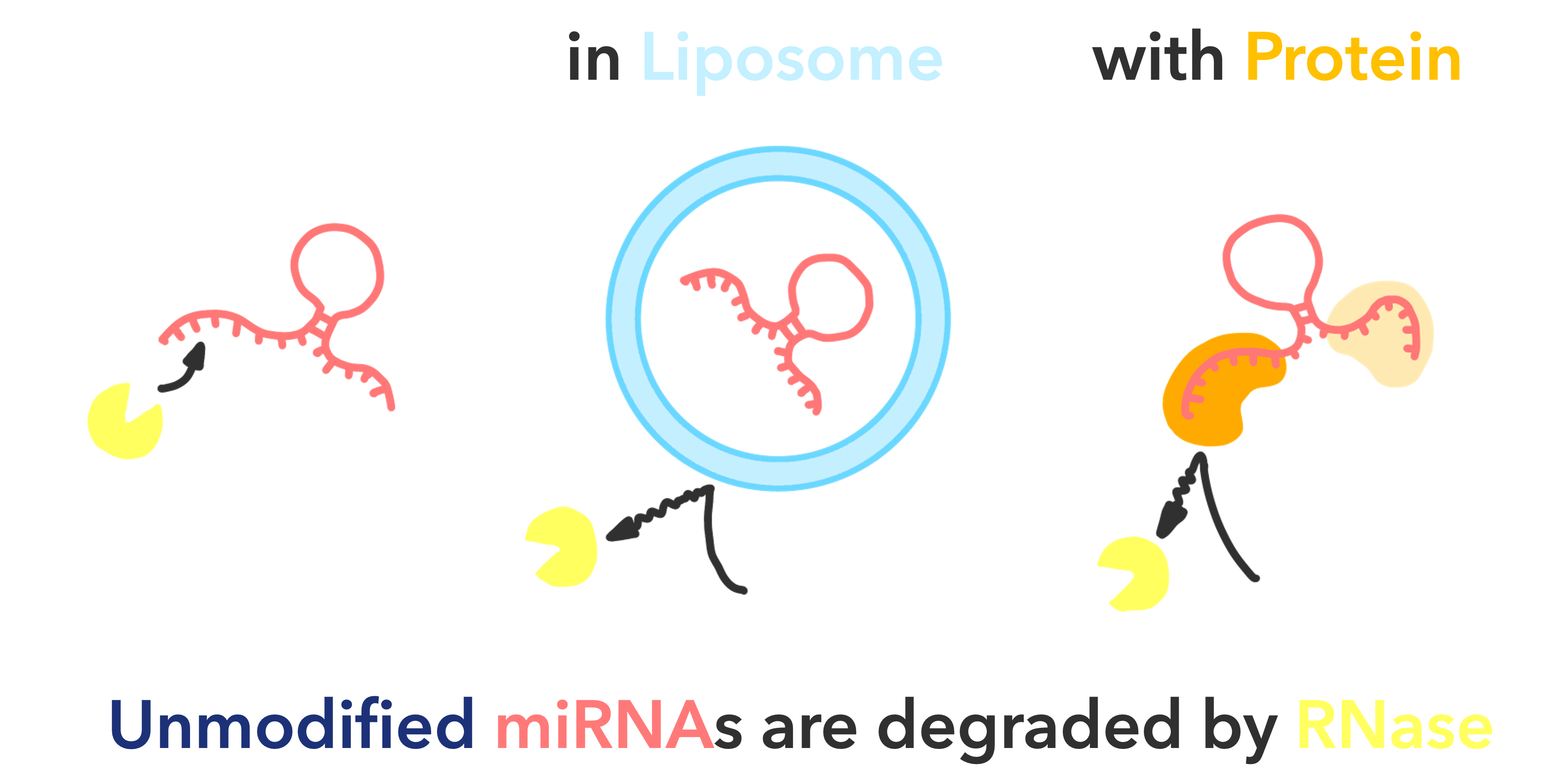
Fig.3 Unmodified miRNA
"Nano Pill Bug"
Our proposed “Nano Pill Bug” is a molecular machine designed to capture unmodified miRNAs and further protect them from RNase. DNA Origami is a structure designed to protect ssRNA from degradation by RNase.
The Nano Pill Bug is a molecular machine that focuses on the speed of miRNA capture and protection from RNase. We came up with the idea of using the bending stress of DNAorigami to capture the miRNAs like a mousetrap. We also named the machine “Nano Pill Bug” because of the way it curls up when it captures and protects the miRNAs, comparing it to a pill bug.
Our proposed “Nano Pill Bug” is a molecular machine designed to efficiently capture unmodified miRNAs and protect them from RNase. Although DNA Origami has been developed in the past to protect ssRNA, there is a limited application of DNA origami technology specifically designed for the rapid capture of miRNAs. The “Nano Pill Bug” offers a new approach that combines both speed of capture and protection.
The “Nano Pill Bug” has a “mousetrap” like structure that captures miRNAs by applying bending stress to the DNA Origami structure. This structure deforms and curls upon contact with the miRNA, effectively capturing and protecting the miRNA. Furthermore, the curling behavior after capture also plays a role in blocking RNase attacks from the outside. We named it “Nano Pill Bug” because its curling behavior, which plays the role of capturing and protecting miRNAs, resembles the curling behavior of a pill bug.
Fig.4 Nano Pill Bug capture and protect miRNA
GOAL
To complete "Nano Pill Bug" Project, we set the following Goals.
- Design of "Nano Pill Bug", a single rounded sheet structure.
- Simulation of "Nano Pill Bug".
- Synthesis of "Nano Pill Bug".
- Design of a pair of "Nano Pill Bug", 2-sheet structure.
- Simulation of a pair of "Nano Pill Bug".
- Simulation of miRNA molecules responding to signals.
- Design of a pair of "Nano Pill Bug".
- Synthesis of a pair of "Nano Pill Bug".
FUTURE
The "Nano Pill Bug" has the ability to capture and protect unmodified miRNAs. With further improvements, it could be used in the field as a diagnostic device for various genetic diseases such as cancer. In addition, it would also be interesting to detect specific unmodified miRNAs and release drugs or, in cooperation with the larger "Micro Pill Bug", to attack the cancer cells themselves.
The concept of utilizing bending stress for capture and protection can be scaled and adapted for use in devices of various sizes. The mechanism of "curling by DNAorigami's own bending stress" offers the possibility of application as a component of molecular robots.
By changing size, shape, target substance (protein, drug, metal), working location, or adding reversibility, the “Nano Pill Bug” will “evolve” to become just like a Pill Bug and be used all over the world.
- [1]Abdelaal, A.M., Sohal, I.S., Iyer, S., et al. 2023. A first-in-class fully modified version of miR-34a with outstanding stability, activity, and anti-tumor efficacy. Oncogene, 42, pp. 2985-2999.
- [2]Ahmed M Abdelaal, Andrea L Kasinski, Ligand-mediated delivery of RNAi-based therapeutics for the treatment of oncological diseases, NAR Cancer, Volume 3, Issue 3, September 2021, zcab030, https://doi.org/10.1093/narcan/zcab030
- [3]Kim, T. & Croce, C.M. 2023. MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies. Experimental & Molecular Medicine, 55, pp. 1314-1321.
- [4]Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009 Dec 1;27(34):5848-56. doi: 10.1200/JCO.2009.24.0317. Epub 2009 Nov 2. PMID: 19884536; PMCID: PMC2793003.
- [5]Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843-54. doi: 10.1016/0092-8674(93)90529-y. PMID: 8252621.
- [6]Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993 Dec 3;75(5):855-62. doi: 10.1016/0092-8674(93)90530-4. PMID: 8252622.
- [7]Pasquinelli, A.E., Reinhart, B.J., Slack, F., et al. 2000. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature, 408, pp. 86-89.